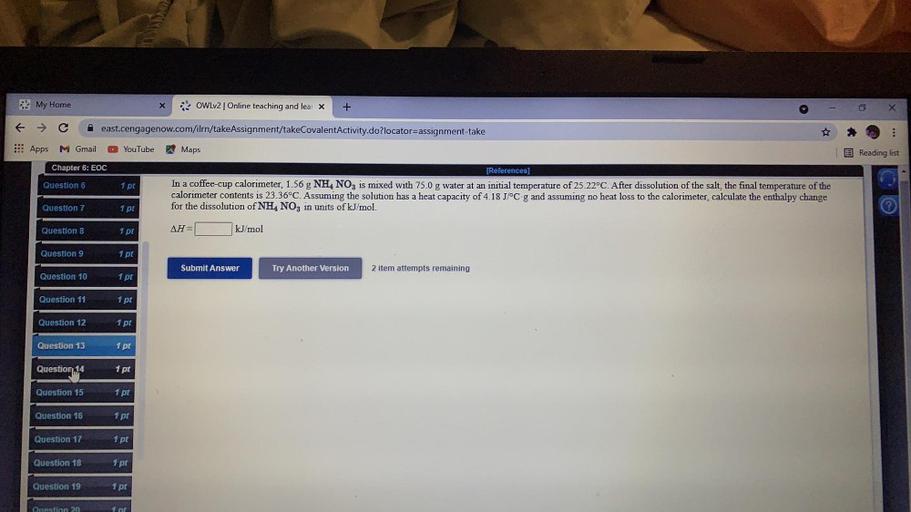
In a coffee-cup calorimeter, 1.91 g of NH4NO3 is mixed with 75.0 g of water at an initial temperature of 25.00 degree Celsius. After the solution of the salt, the final temperature was 20.94 degree Ce; In a coffee cup calorimeter, 1.17 grams of NH4NO3 was mixed with 55.00 grams of water at an initial temperature of 25.00 degrees Celsius.. In a coffee-cup calorimeter, 1.60 gNH4NO3 is mixed with 75.0 g water at an initial temperature of 25.00^∘C. After dissolution of the salt, the final temperature of the calorimeter contents is 23.34^∘C.. calculate the enthalpy change for the dissolution of NH4NO3 in units of kJ / mol. In a coffee-cup calorimeter, $1.60 \mathrm{~g} \mathrm.
Identify What a Coffee Cup Calorimeter Measures.
PPT TOPIC 8 THERMOCHEMISTRY PowerPoint Presentation, free download ID3087554

SOLVEDA 14.1mL sample of 0.996 M NaOH is mixed with 32.3 mL of 0.905 M HCl in a coffeecup
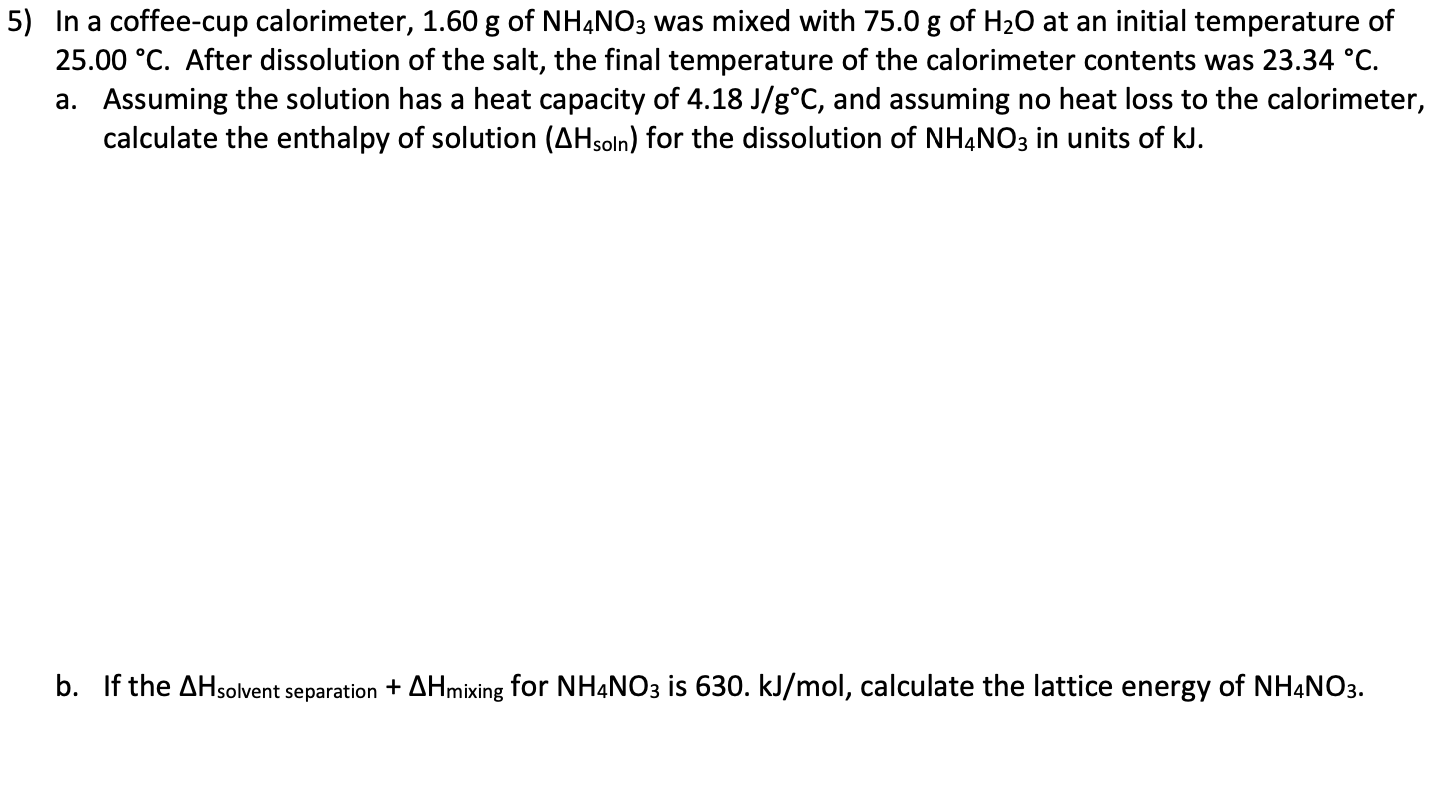
Solved 5) In a coffeecup calorimeter, 1.60 g of NH4NO3 was
SOLVED A 50.0mL sample of 0.250 M HCl is added to a 50.0mL sampleof 0.250 M NaOH in a
My Home x OWLv2 Online teaching and lear... Chemistry

PPT Thermochemistry PowerPoint Presentation, free download ID1552140
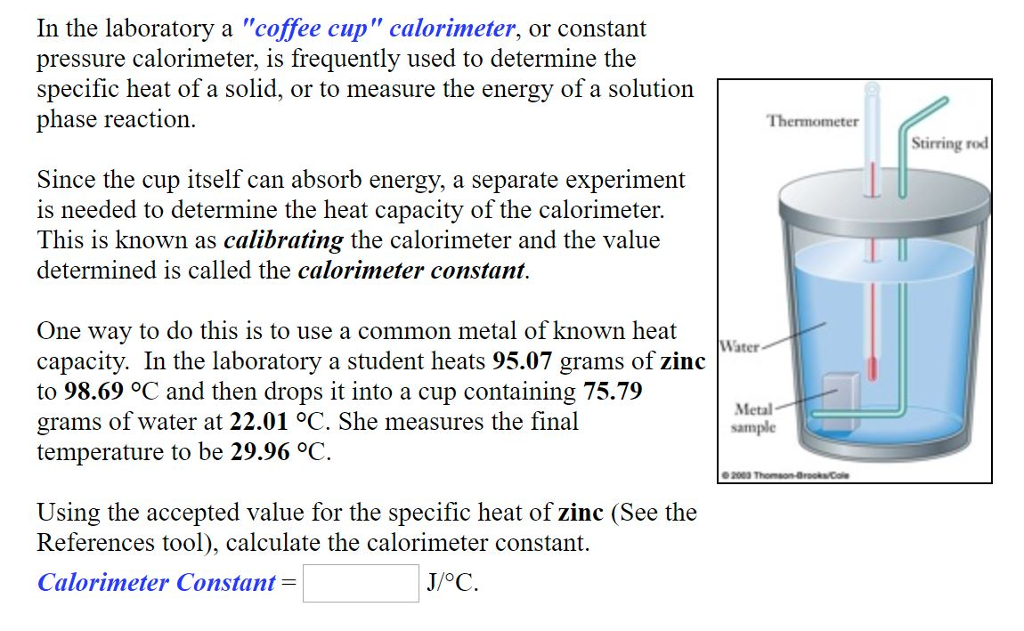
Solved In the laboratory a "coffee cup" calorimeter, or
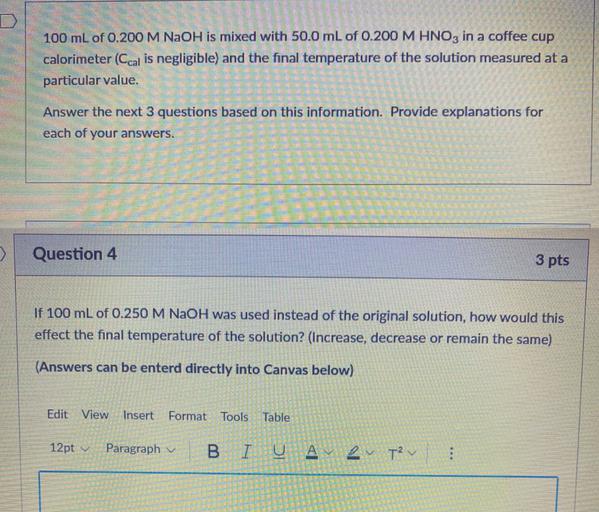
D 100 mL of 0.200 M NaOH is mixed with 50... Physical Chemistry

Enthalpy changes in solution
/GettyImages-141482586-566245ac3df78ce16197a1fd.jpg)
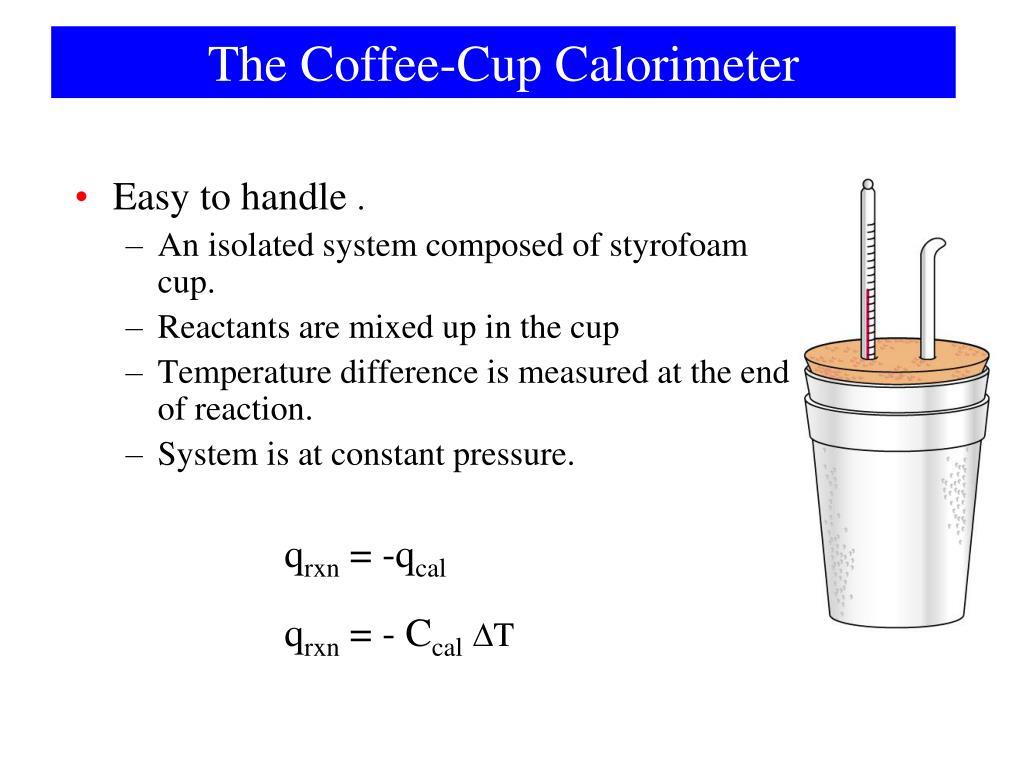
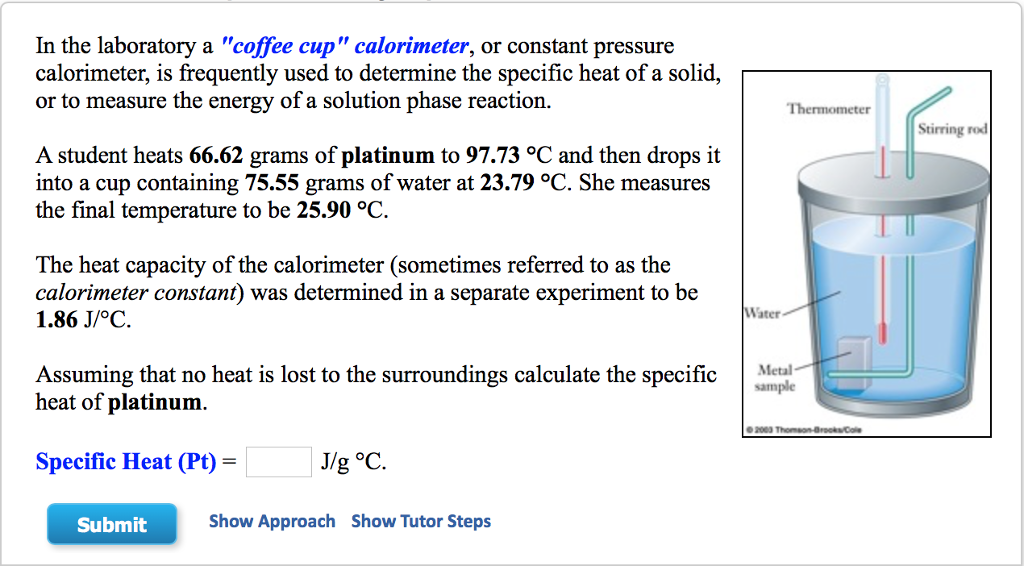
Coffee Cup and Bomb Calorimetry
![[Solved] In a coffeecup calorimeter, ( 140.0 mathrm{mL [Solved] In a coffeecup calorimeter, ( 140.0 mathrm{mL](https://media.cheggcdn.com/study/97a/97a6a2ed-4345-413a-9790-752875fc2d9e/image)
[Solved] In a coffeecup calorimeter, ( 140.0 mathrm{mL
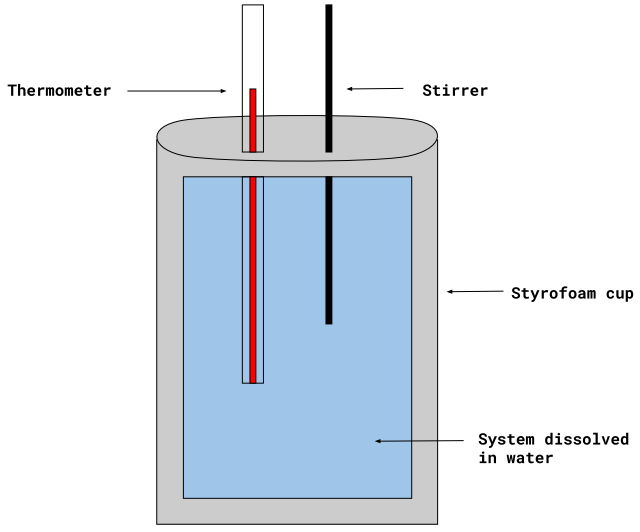
Understanding Coffee Cup Calorimetry

SOLVED In a coffeecup calorimeter, 1.70 g of NH4NO3 is mixed with 72.0 g of water at an
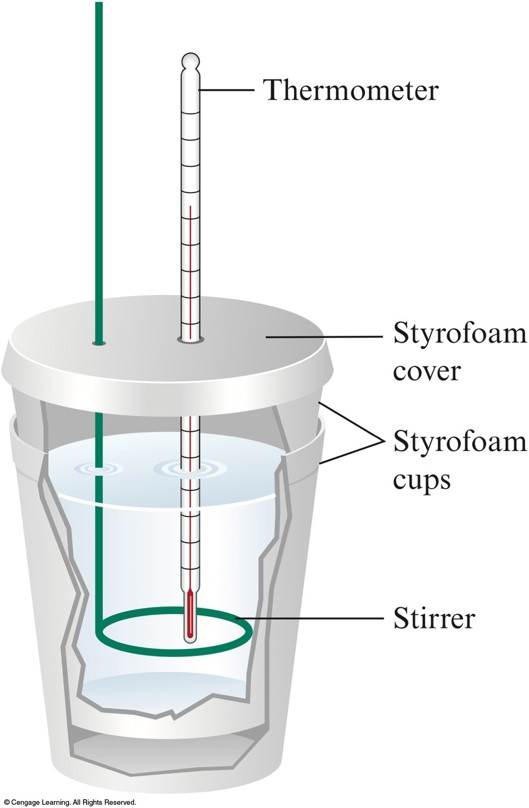
Chapter 5 Presentation

Solved In the laboratory a "coffee cup" calorimeter, or
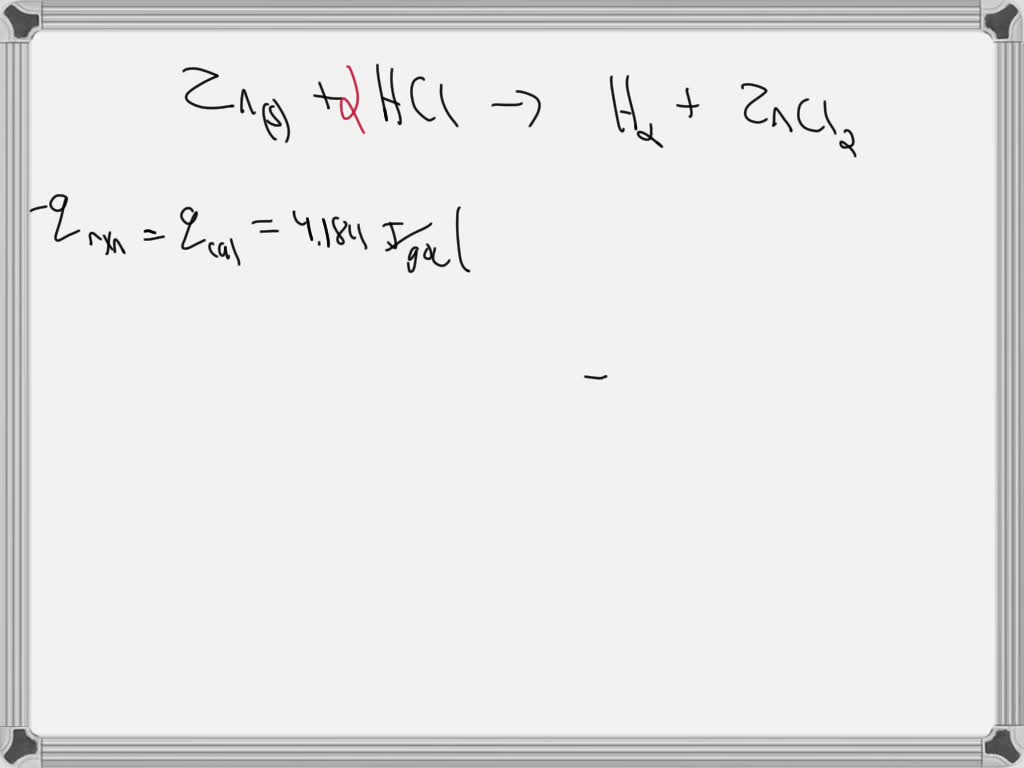
SOLVED A coffeecup calorimeter contains 100.0 mL of 0.300 M HCl at 20.3 °C. When 1.82 g Zn(s

32+ calorimeter constant calculator RachaelOcean

Pin on All About Science

The Difference Between Calories in Foodstuff and Calories in Combustible Materials
In a coffee cup calorimeter, 1.60 g of NH4NO3 is mixed with 75.0 g of water at an initial temperature of 25.008C. After dissolution of the salt, the final temperature of the calorimeter contents is 23.348C. Assuming the solution has a heat capacity of 4.18 J 8C21 g21 and assuming no heat loss to the calorimeter, calculate the enthalpy change.. Question. In a coffee cup calorimeter, 1.60 g of \mathrm { NH } _ { 4 } \mathrm { NO } _ { 3 } NH4NO3 is mixed with 75 .0 g of water at an initial temperature of 25.00^\circ C. 25.00∘C. After dissolution of the salt, the final temperature of the calorimeter content s is 23.34^\circ C. 23.34∘C.